About Us
Business Model/Overview
Business Model
- GNI Group Ltd is a vertically integrated multinational bio-pharma company, comprised of drug research, clinical development, manufacturing, sales and marketing.
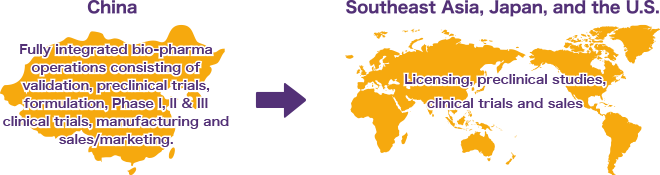
- The Company’s base of operations is in China, enabling it to leverage that country’s comparative cost advantage in clinical trials to develop Class 1 “First to Market” drug products for the Chinese domestic market.
- Such drugs can then be migrated to other markets through out-licensing, co-development and collaborative agreements to expand the Company’s global revenue sources.
Business Overview
Business Overview of the GNI Group Ltd.
The Company seeks to leverage its competitive advantages to enhance the probability for the successful and accelerated development of new drug products, with the primary objective to reach more patients with new therapies.
The Company’s drug development focuses on orphan diseases where the patient needs are most urgent, utilizing “Fast-track” regulatory status and smaller size clinical trials requirements to meet such urgent therapy needs at reasonable pricing.
The Company’s strategy is to broaden the scope of its approved drug products to deliver therapy additional treatment to more patients. As a first step, we pursue development of Class I drugs for diseases without proven therapies. Second, we seek to broaden the usage of such drugs for new indication therapies to treat larger patient populations and build a strong network among China’s leading hospitals and KOLs for the treatment of such diseases. As a third step, we intend to expand these drugs into the international markets through out-licensing and collaborations.
We continue to look to grow and optimize our drug pipeline to take advantage of the lower costs for clinical trials in China (less than 10% of comparable costs in Japan, Europe and United States) via license-in opportunities of new drugs from outside China. Moreover, we will pursue licensing-out and partnership opportunities in US/EU/Japan using our F351 drug product in its advanced stage of development.
Main features of GNI Group
- Hold several candidate compounds in our drug discovery pipeline, and develop therapeutic agents that target diseases prevalent in Japan, China, and other Asian countries.
- Own our own pharmaceutical factory in China, enabling us to conduct an integrated set of business operations ranging from the search for new drugs to their clinical development, manufacture, and sales.
GNI Group’s drug discovery activities
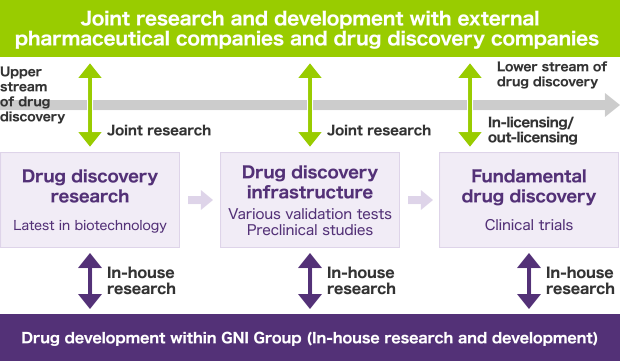
GNI Group’s business operations consist of the three following drug discovery activities.
Drug discovery research
We utilize the latest in biotechnology to exclusively (or jointly with external pharmaceutical companies through collaborative research) identify the target genes or action mechanisms. We also conduct function estimation on unknown genes.
Drug discovery infrastructure
We exclusively (or jointly with external pharmaceutical companies through collaborative research) conduct various validation tests and preclinical studies.
Fundamental drug discovery
We conduct clinical trials on candidate compounds developed exclusively by GNI Group (or licensed external candidate compounds), obtain approval for drugs, and plan and undertake their manufacture and sales.
Drug discovery approaches employed by GNI Group
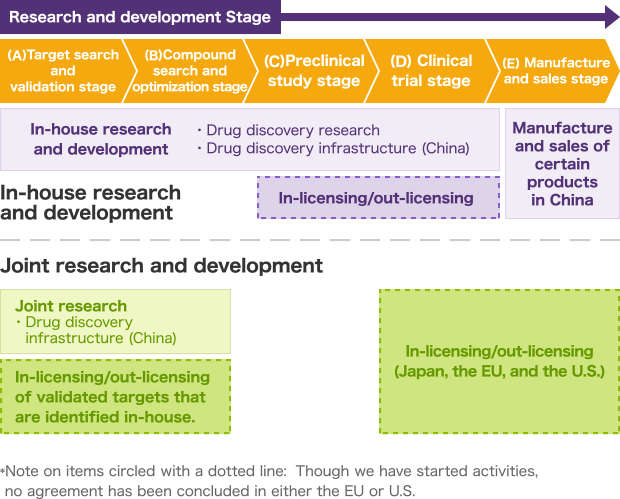
GNI Group takes two main approaches for the three drug discovery activities previously mentioned: in-house research and development, and joint research and development. These two approaches, which are described in greater detail in the chart below, each consists of five stages: (A) Target search and validation, (B) Compound search and optimization, (C) Preclinical study, (D) Clinical trial, and (E) Manufacturing and sales.
For in-house research and development, we handle everything from the target search and validation stage (A) to the clinical trial stage (D) shown in the chart below as part of the integrated set of business operations of GNI Group. We established Beijing Continent Pharmaceutical Co, Ltd. as a subsidiary company to realize the manufacture and sales stage (E) for Etuary (艾思瑞® in Chinese), which is a drug approved for use as a therapeutic agent for idiopathic pulmonary fibrosis (IPF). We filed an application for the manufacture and sales of this drug in February 2013, obtained approval in December of the same year, and then began sales in February 2014.
In the area of joint research and development, our main strength lies in the target search and validation stage (A). We conduct research projects in China with leading pharmaceutical companies from around the world, utilizing the latest in biotechnology within our work.